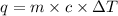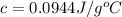You placed 43.1 g of an unknown metal at 100 °c into a coffee cup calorimeter that contained 50.0 g of water that was initially at 22.0 °c. the equilibrium temperature of mixing (t0) was determined to be 23.2 °c. the calorimeter constant was known to be 51.5 j/°c. specific heath2o = 4.184 j/g·°ca. what is the total amount of heat (j) lost by the metal? ng 1.5b. what was the specific heat (j/g·°c) of the metal? ng 1.5

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


Do you know the correct answer?
You placed 43.1 g of an unknown metal at 100 °c into a coffee cup calorimeter that contained 50.0 g...
Questions in other subjects:

Biology, 25.01.2021 23:00





Mathematics, 25.01.2021 23:00





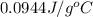
![q=[q_1+q_2]](/tpl/images/0381/4030/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0381/4030/1d50b.png)
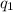 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter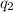 = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 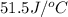
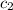 = specific heat of water =
= specific heat of water = 
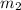 = mass of water = 50.0 g
= mass of water = 50.0 g = change in temperature =
= change in temperature = 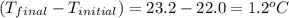
![q=[(51.5J/^oC\times 1.2^oC)+(50.0g\times 4.184J/g^oC\times 1.2^oC)]](/tpl/images/0381/4030/aca3d.png)