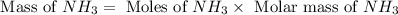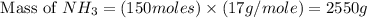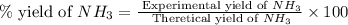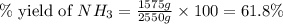Chemistry, 19.11.2019 06:31, jlbradley429
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with nitrogen, 1575 g of ammonia were produced. what is the percent yield of this reaction?
30.8%
61.8%
20.7%
41.5%
more information is needed to solve this problem.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2



Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
Do you know the correct answer?
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with...
Questions in other subjects:


Computers and Technology, 07.07.2021 08:40

English, 07.07.2021 08:40


Social Studies, 07.07.2021 08:50

Biology, 07.07.2021 08:50

English, 07.07.2021 08:50


Mathematics, 07.07.2021 08:50


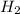 = 450 g
= 450 g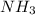 = 17 g/mole
= 17 g/mole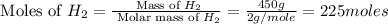
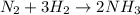
 moles of
moles of