
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. chlorine trifluoride is prepared by the reaction cl2 (g) 3f2 (g) ⟶ 2clf3 (g). write the equation that relates the rate expressions for this reaction in terms of the disappearance of cl2 and f2 and the formation of clf3.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
Do you know the correct answer?
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile co...
Questions in other subjects:


Mathematics, 07.12.2021 01:00



Health, 07.12.2021 01:00





Mathematics, 07.12.2021 01:00


![Rate=-\frac{d[Cl_2]}{dt}=-\frac{1}{3}\frac{d[F_2]}{dt}=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/d2692.png)
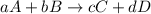
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0380/6825/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0380/6825/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0380/6825/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/d4b94.png)
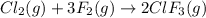
![\text{Rate of disappearance of }Cl_2=-\frac{d[Cl_2]}{dt}](/tpl/images/0380/6825/4403e.png)
![\text{Rate of disappearance of }F_2=-\frac{1}{3}\frac{d[F_2]}{dt}](/tpl/images/0380/6825/c24a1.png)
![\text{Rate of formation of }ClF_3=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/1400a.png)




