Chemistry, 19.11.2019 02:31, kaylallangari2145
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are heated together. if 20.0 g of hydrogen and 20.0 g of iodine are heated, forming 10.0 g of hydrogen iodide, what mass of hydrogen remains unreacted? a. 10.0 g hydrogen remains b. 10.9 g hydrogen remains c. 15.0 g hydrogen remains d. 19.9 g hydrogen remains.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1



Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
Do you know the correct answer?
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are h...
Questions in other subjects:











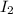
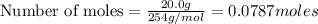
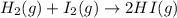
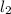 require=
require=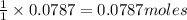 of
of