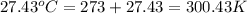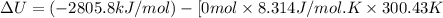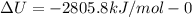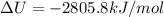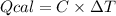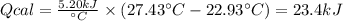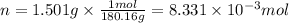Chemistry, 19.11.2019 02:31, Camill0310
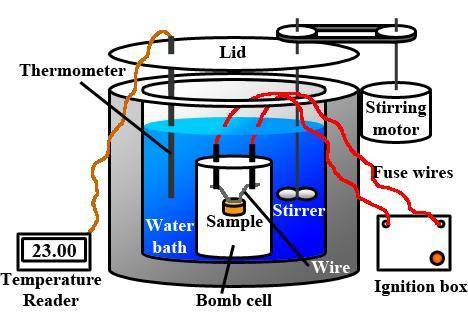
The combustion of 1.5011.501 g of fructose, c6h12o6(s)c6h12o6(s) , in a bomb calorimeter with a heat capacity of 5.205.20 kj/°c results in an increase in the temperature of the calorimeter and its contents from 22.9322.93 °c to 27.4327.43 °c. what is the internal energy change, δδu , for the combustion of 1.5011.501 g of fructose?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3


Chemistry, 23.06.2019 05:30, victoria6929
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
Do you know the correct answer?
The combustion of 1.5011.501 g of fructose, c6h12o6(s)c6h12o6(s) , in a bomb calorimeter with a heat...
Questions in other subjects:


Chemistry, 25.09.2019 02:10



Chemistry, 25.09.2019 02:10




Mathematics, 25.09.2019 02:10

English, 25.09.2019 02:10



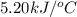
 = final temperature =
= final temperature = 
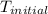 = initial temperature =
= initial temperature = 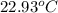



 = enthalpy change = ?
= enthalpy change = ?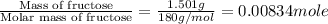

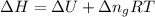


 = change in internal energy = ?
= change in internal energy = ? = change in moles = 0 (from the reaction)
= change in moles = 0 (from the reaction)