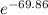Chemistry, 19.11.2019 02:31, valoiserika1229
For the reaction of oxygen and nitrogen to form nitric oxide, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh⁰rxn 180.5kj/mol δs⁰rxn 24.80j/(mol⋅k) a. calculate the temperature in kelvins above which this reaction is spontaneous b. the thermodynamic values from part a will be useful as you work through part b: δh⁰rxn 180.5kj/mol δs⁰rxn 24.80j/(mol⋅k) calculate the equilibrium constant for the following reaction at room temperature, 25 ∘c : n2(g)+o2(g)→2no(g)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
Do you know the correct answer?
For the reaction of oxygen and nitrogen to form nitric oxide, consider the following thermodynamic d...
Questions in other subjects:

Mathematics, 26.06.2020 16:01




Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01


Business, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01