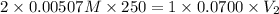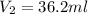Chemistry, 19.11.2019 01:31, fluffylove83
Achemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. a. calculate the volume of solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
Do you know the correct answer?
Achemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilut...
Questions in other subjects:

Medicine, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40


Law, 27.10.2020 21:40

English, 27.10.2020 21:40



Mathematics, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40


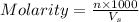
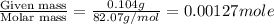
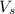 = volume of solution in ml
= volume of solution in ml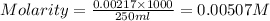
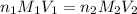
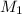 = molarity of
= molarity of 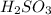 solution = 0.00507 M
solution = 0.00507 M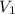 = volume of
= volume of 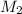 = molarity of
= molarity of 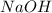 solution = 0.0700 M
solution = 0.0700 M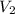 = volume of
= volume of 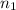 = valency of
= valency of 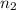 = valency of
= valency of