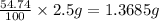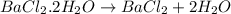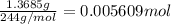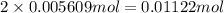Chemistry, 19.11.2019 01:31, sixtomomtermont
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the mixture weighed 72.248g. when empty the beaker weighed 69.748g. the mixture was determined to contain 45.26% sand.
(a) what is the % and mass of the hydrate in the mixture?
(b) if the mixture was selectively decomposed by heating, how many grams and moles of water would be lost?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the m...
Questions in other subjects:


Mathematics, 20.05.2020 05:59


Mathematics, 20.05.2020 05:59




Mathematics, 20.05.2020 05:59


Mathematics, 20.05.2020 05:59