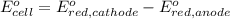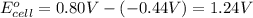Chemistry, 19.11.2019 01:31, burnsmykala23
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given that the standard reduction potential of ag+ to ag (s) is +0.80 v and the standard reduction potential of fe2+ to fe (s) is −0.44 v, calculate the standard cell potential, e°cell.−1.24 v1.24 v2.04 v0.36 v

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, sanchezvazquez0123
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
Do you know the correct answer?
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given...
Questions in other subjects:

Mathematics, 12.03.2021 20:30

Mathematics, 12.03.2021 20:30


Mathematics, 12.03.2021 20:30

Mathematics, 12.03.2021 20:30






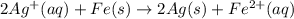
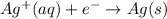
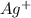 to Ag=
to Ag=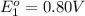
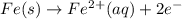
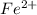 to Fe=
to Fe=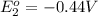
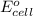 of the reaction, we use the equation:
of the reaction, we use the equation: