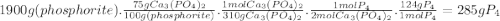Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c → 6casio3+p4+10co phosphorite is a mineral that contains ca3(po4)2 by mass? assume an excess of the other reactants plus other non-phosphorus-containing compounds. what is the maximum amount of p4 that can be produced from 1.9kg of phosphorite sample is 75% ca3(po4)2 by mass? assume an excess of the other reactants

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c →...
Questions in other subjects:

Mathematics, 02.04.2021 19:40






Physics, 02.04.2021 19:40