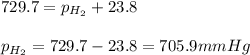Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochloric acid and trapping the gas produced in an inverted tube initially filled with water. if the total pressure of the gas in the collection tube is 729.7 mmhg at 25°c, what is the partial pressure of the hydrogen? the vapor pressure of water is 23.8 mmhg.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochlor...
Questions in other subjects:

History, 21.11.2020 18:40






English, 21.11.2020 18:40

Chemistry, 21.11.2020 18:40

Mathematics, 21.11.2020 18:40


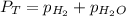
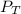 = 729.8 mmHg
= 729.8 mmHg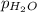 = 23.8 mmHg
= 23.8 mmHg