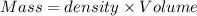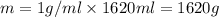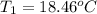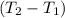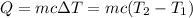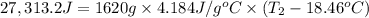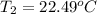Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of both solutions is the same at 18.46°c. the heat of neutralization when 1.00 mol of hno3 reacts with 0.500 mol ba(oh)2 is −56.2 kj/mol. assume that the densities and specific heats of the solution are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively). what is the final temperature of the solution?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
Do you know the correct answer?
Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a const...
Questions in other subjects:

Mathematics, 05.01.2021 22:30


Mathematics, 05.01.2021 22:30

Mathematics, 05.01.2021 22:30

Mathematics, 05.01.2021 22:30





Mathematics, 05.01.2021 22:30


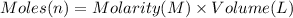
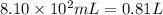
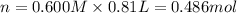
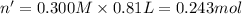
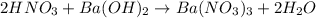
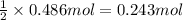 barium hydroxide.
barium hydroxide.