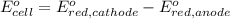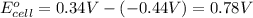In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are cu2+(aq)+2e−→cu(s) and fe(s)→fe2+(aq)+2e− the net reaction is cu2+(aq)+fe(s)→cu(s)+fe2+(aq) use the given standard reduction potentials in your calculation as appropriate.

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions in other subjects:

Mathematics, 16.02.2021 02:50


History, 16.02.2021 02:50

History, 16.02.2021 02:50

Mathematics, 16.02.2021 02:50

Mathematics, 16.02.2021 02:50


Computers and Technology, 16.02.2021 02:50



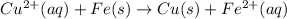
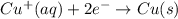
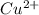 to Cu=
to Cu=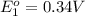
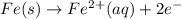
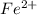 to Fe=
to Fe=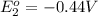
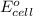 of the reaction, we use the equation:
of the reaction, we use the equation: