
Chemistry, 18.11.2019 20:31, Thunderalesis7855
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solution. first the student prepares a set of cocl2(aq) solutions of known concentration. then the student uses a spectrophotometer to determine the absorbance of each of the standard solutions at a wavelength of 510nm and constructs a standard curve. finally, the student determines the absorbance of the sample of unknown concentration. a wavelength of 510nm corresponds to an approximate frequency of 6×1014s−1. what is the approximate energy of one photon of this light? 9×1047j.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Do you know the correct answer?
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solu...
Questions in other subjects:


Biology, 28.09.2021 23:10

Geography, 28.09.2021 23:10


Biology, 28.09.2021 23:10


Computers and Technology, 28.09.2021 23:10


English, 28.09.2021 23:10

Arts, 28.09.2021 23:10


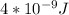
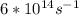
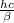 ---- ( 1 )
---- ( 1 )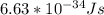
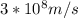
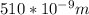
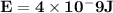
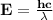
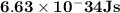
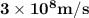
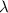 - wavelength = 510nm
- wavelength = 510nm



