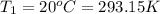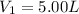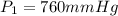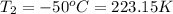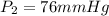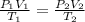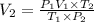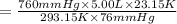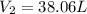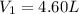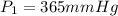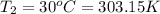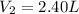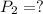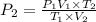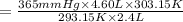Chemistry, 18.11.2019 20:31, PBWaffles4864
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 2.04×108 pa , what is its running pressure in torr?
b)a sample of gas in a balloon has an initial temperature of 37 ∘c and a volume of 1.12×103 l . if the temperature changes to 58 ∘c , and there is no change of pressure or amount of gas, what is the new volume, v2, of the gas?
c)a very flexible helium-filled balloon is released from the ground into the air at 20. ∘c. the initial volume of the balloon is 5.00 l, and the pressure is 760. mmhg. the balloon ascends to an altitude of 20 km, where the pressure is 76.0 mmhg and the temperature is −50. ∘c. what is the new volume, v2, of the balloon in liters, assuming it doesn't break or leak?
d )consider 4.60 l of a gas at 365 mmhg and 20. ∘c . if the container is compressed to 2.40 l and the temperature is increased to 30. ∘c , what is the new pressure, p2, inside the container? assume no change in the amount of gas inside the cylinder.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
Do you know the correct answer?
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify...
Questions in other subjects:



Mathematics, 18.03.2021 02:30


Physics, 18.03.2021 02:30




Mathematics, 18.03.2021 02:30


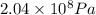
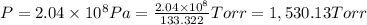
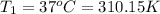
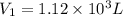
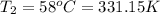
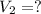
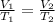 (constant pressure)
(constant pressure)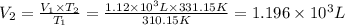
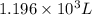 is the new volume of the gas.
is the new volume of the gas.