
Chemistry, 18.11.2019 19:31, mixedgirlmara
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6 kj / mol rxn. using the following standard enthalpy of formation data, calculate standard enthalpy of formation for sic (s). a. standard enthalpy of formation sio2 (s) = -910.9 kj/mol b. standard enthalpy of formation co (g) = -110.5 kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, annarain2004
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
Do you know the correct answer?
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6...
Questions in other subjects:


History, 04.02.2021 05:50




Mathematics, 04.02.2021 05:50

Biology, 04.02.2021 05:50



History, 04.02.2021 05:50


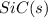 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol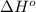
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0379/7554/72c39.png)
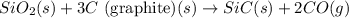
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times \Delta H^o_f_{(CO(g))})]-[(1\times \Delta H^o_f_{(SiO_2(s))})+(3\times \Delta H^o_f_{(C(s))})]](/tpl/images/0379/7554/6a7fe.png)
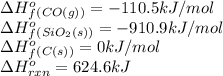
![624.6=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times (-110.5))]-[(1\times (-910.9))+(3\times (0))]\\\\\Delta H^o_f_{(SiC(s))}=-65.3kJ/mol](/tpl/images/0379/7554/c8e18.png)




