Chemistry, 18.11.2019 19:31, brittanyfox411
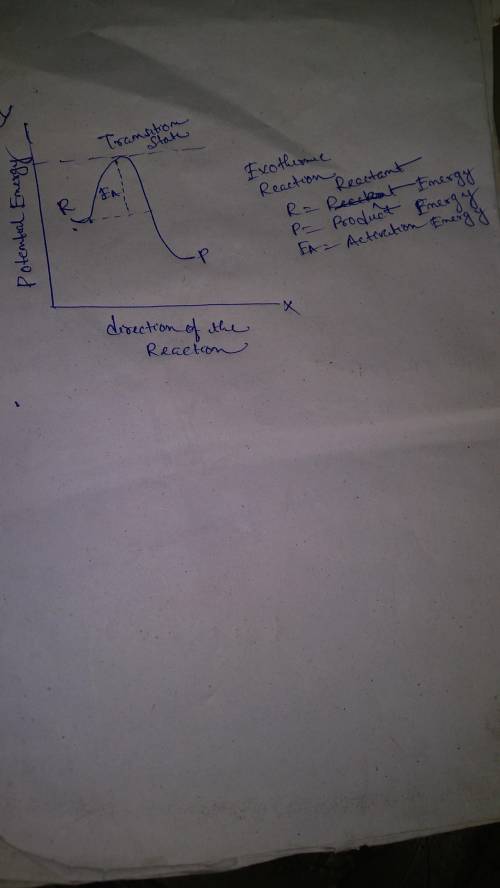
Which statement is true?
in an endothermic reaction, the energy of the products is the same as the energy of the reactants.
in an endothermic reaction, the energy of the products is less than the energy of the reactants.
in an exothermic reaction, the energy of the products is less than the energy of the reactants.
in an exothermic reaction, the energy of the products is the same as the energy of the reactants.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
Do you know the correct answer?
Which statement is true?
in an endothermic reaction, the energy of the products is the...
in an endothermic reaction, the energy of the products is the...
Questions in other subjects:

Mathematics, 06.08.2019 22:10