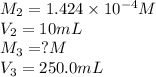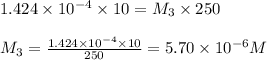Chemistry, 18.11.2019 18:31, sandeebassett3
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions instead of measuring a very small mass. a solution was prepared by dissolving 0.360 g of kno3 in enough water to make 500. ml of solution. a 10.0 ml sample of this solution was transferred to a 500.0-ml volumetric flask and diluted to the mark with water. then 10.0 ml of the diluted solution was transferred to a 250.0-ml flask and diluted to the mark with water. what is the final concentration of the kno3 solution? 7.91 × 10-9 m1.42 × 10-4 m5.70 × 10-6 m2.85 × 10-6 m7.12 × 10-3 m

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
Do you know the correct answer?
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions...
Questions in other subjects:





Mathematics, 27.08.2021 16:40




Mathematics, 27.08.2021 16:40

Mathematics, 27.08.2021 16:40


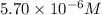
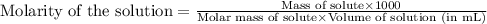
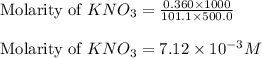
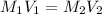 .......(1)
.......(1)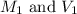 are the molarity and volume of the concentrated
are the molarity and volume of the concentrated 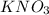 solution
solution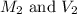 are the molarity and volume of diluted
are the molarity and volume of diluted 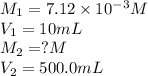
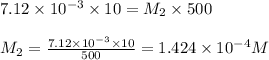
 are the molarity and volume of diluted
are the molarity and volume of diluted