
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures the evolution of 670 j of heat as the two substances react to give cu3(po4)2(s). compute the δh that would result from the reaction of 1.00 mo! cucl2 with an excess of (nh4)2hpo4.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
Do you know the correct answer?
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures th...
Questions in other subjects:




Mathematics, 26.10.2020 19:20


History, 26.10.2020 19:20

Chemistry, 26.10.2020 19:20




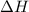 will be 90054 J
will be 90054 J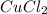 = 134.45 g/mol
= 134.45 g/mol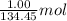 of
of 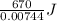 of heat or 90054 J of heat
of heat or 90054 J of heat



