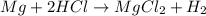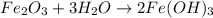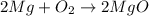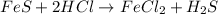Which of the following is a single replacement reaction? fe2o3 + 3h2o yields 2fe(oh)3
fes + 2h...

Chemistry, 25.08.2019 15:20, myiacoykendall
Which of the following is a single replacement reaction? fe2o3 + 3h2o yields 2fe(oh)3
fes + 2hcl yields fecl2 + h2s
2mg + o2 yields 2mgo
mg + 2hcl yields mgcl2 + h2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, muncyemily
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
Do you know the correct answer?
Questions in other subjects:


English, 01.10.2019 13:10

English, 01.10.2019 13:10



English, 01.10.2019 13:10

Business, 01.10.2019 13:10

Computers and Technology, 01.10.2019 13:10