
Chemistry, 16.11.2019 07:31, 20jessicar598
The equilibrium constant for the dissolution of silver chloride (agcl(s) ag+(aq) + cl–(aq)) has a value of 1.79 × 10–10. which statement is true?
a. silver chloride is completely soluble in water.
b. silver chloride is essentially insoluble in water.
c. no right choice.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2


Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
Do you know the correct answer?
The equilibrium constant for the dissolution of silver chloride (agcl(s) ag+(aq) + cl–(aq)) has a va...
Questions in other subjects:



Mathematics, 01.06.2021 21:00

Mathematics, 01.06.2021 21:00


Mathematics, 01.06.2021 21:00



Mathematics, 01.06.2021 21:00


 for silver chloride is determined by equilibrium concentrations of dissolved ions. But solubility may vary also at different temperatures. Complete solubility is possible in ammonia solution as it form stable complex as water is not good ligand for Ag+.
for silver chloride is determined by equilibrium concentrations of dissolved ions. But solubility may vary also at different temperatures. Complete solubility is possible in ammonia solution as it form stable complex as water is not good ligand for Ag+. 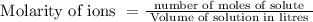
![\left[\mathbf{A} \mathbf{g}^{+}\right]=[\mathbf{C} \mathbf{I}]=\text { molarity of ions }](/tpl/images/0377/2340/63684.png)
![\left[\mathbf{A} \mathbf{g}^{+}\right] \cdot[\mathbf{C} \mathbf{I}]](/tpl/images/0377/2340/66898.png)




