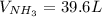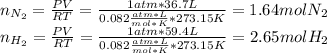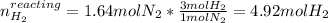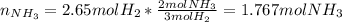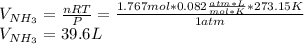Chemistry, 16.11.2019 03:31, kedjenpierrelouis
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh3(g). what volume of ammonia (in l) could be produced by the reaction of 59.4 liters of hydrogen with 36.7 liters of nitrogen at a constant pressure and temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1


Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
Do you know the correct answer?
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh...
Questions in other subjects:

Geography, 19.11.2020 16:20

History, 19.11.2020 16:20



Mathematics, 19.11.2020 16:20

Spanish, 19.11.2020 16:20

Mathematics, 19.11.2020 16:20

Biology, 19.11.2020 16:20