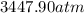Chemistry, 16.11.2019 03:31, prettyluhangel
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by the van der waals equation? the ideal gas law constant is 0.08206 [l•atm] / [mol•k]. for co2, the pressure correction constant is 3.658 l2•atm / mol 2, and the volume correction constant is 0.04286 l / mol.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
Do you know the correct answer?
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by th...
Questions in other subjects:

Arts, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31

History, 13.12.2019 19:31

Physics, 13.12.2019 19:31






Mathematics, 13.12.2019 19:31


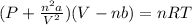
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}][1.0L-(20.0mol\times0.04286 L.mol^{-1} )]=(20.0mol)\times (0.08206L.atm.mol^{-1}.K^{-1})\times (300.0K)](/tpl/images/0376/9858/97073.png)
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}]](/tpl/images/0376/9858/353d5.png) =
=