
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constant kp has a value of 0.003691 based on a reference-state pressure of 1 atm (101,325 pa). derive an algebraic ex- pression for the forward rate coefficient kf . evaluate your expression for a temperature of 1500 k. give units.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constan...
Questions in other subjects:

Mathematics, 23.09.2019 06:20



Mathematics, 23.09.2019 06:20

Mathematics, 23.09.2019 06:20

Chemistry, 23.09.2019 06:20


Mathematics, 23.09.2019 06:20



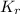 =
= 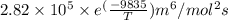
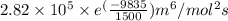
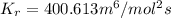
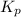 and
and 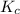 is as follows.
is as follows.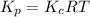
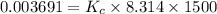
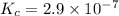
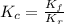
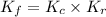
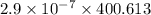
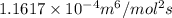
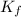 is
is 




