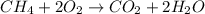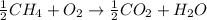Which statement about the following reaction is correct?
ch4 (g) + 2o2 (g) yields co2 (...

Chemistry, 30.01.2020 12:50, dlaskey646
Which statement about the following reaction is correct?
ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o(l) deltah = -890 kj
reacting one mole of oxygen (o2) absorbs 445 kj of energy
reacting one mole of oxygen (o2) releases 445 kj of energy
reacting one mole of methane (ch4) absorbs 890 kj of energy
reacting two moles of methane (ch4) releases 890 kj of energy

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 12:30, bryantjorell
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Arts, 19.01.2021 21:20



Mathematics, 19.01.2021 21:20

Mathematics, 19.01.2021 21:20





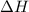 for the reaction comes out to be negative.
for the reaction comes out to be negative.