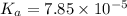Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
Do you know the correct answer?
1.50 g of a weak acid (molar mass 176) is dissolved in 50.0 ml of water, and the resultant solution...
Questions in other subjects:

English, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01


History, 18.10.2020 07:01


Mathematics, 18.10.2020 07:01


Computers and Technology, 18.10.2020 07:01


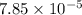 .
.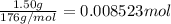
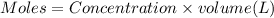
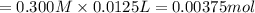
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0376/5102/e4eea.png)

![pH=pK_a+\log(\frac{[NaA]}{[HA]})](/tpl/images/0376/5102/83d67.png)
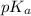 = ?
= ?![[HA]=\frac{0.008523 mol-0.00375}{0.0625 L}=\frac{0.004773 mol}{0.0625 L}](/tpl/images/0376/5102/6fe77.png)
![[NaA]=\frac{0.00375 mol}{0.0625 L}](/tpl/images/0376/5102/93c44.png)
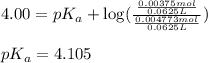
![4.105=-\log[K_a]](/tpl/images/0376/5102/b305f.png)