Chemistry, 15.11.2019 20:31, Mrblunt5613
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g) δh o rxn = −1255.8 kj given δh o f of co2(g) = −393.5 kj/mol and δh o f of h2o(g) = −241.8 kj/mol, find δh o f of c2h2(g).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
Do you know the correct answer?
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g)...
Questions in other subjects:

English, 03.04.2021 18:30

SAT, 03.04.2021 18:30

Mathematics, 03.04.2021 18:30

English, 03.04.2021 18:30

Computers and Technology, 03.04.2021 18:30

Mathematics, 03.04.2021 18:30

English, 03.04.2021 18:30

Mathematics, 03.04.2021 18:30


Computers and Technology, 03.04.2021 18:30


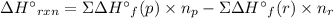
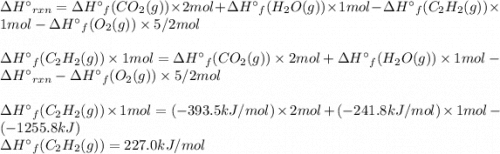
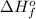 , of C₂H₂ is 227 kJ/mol
, of C₂H₂ is 227 kJ/mol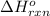 = −1255.8 kJ
= −1255.8 kJ