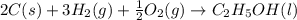Chemistry, 15.11.2019 20:31, johnnybones03
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that represents the formation of liquid ethanol? a. 2 c(s) + 6 h(g) + o(g) → c2h5oh(l) b. 2 c(s) + 3 h2(g) + ½ o2(g) → c2h5oh(l) c. 2co2(g) + 3h2o(g) → c2h5oh(l) + 3 o2(g) d. 4 c(s) + 6 h2(g) + o2(g) → 2 c2h5oh(l)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
Do you know the correct answer?
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that rep...
Questions in other subjects:




History, 07.10.2019 00:00

Mathematics, 07.10.2019 00:00


Mathematics, 07.10.2019 00:00


Geography, 07.10.2019 00:00