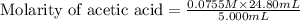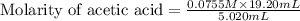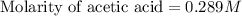Chemistry, 15.11.2019 18:31, beckers0115
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized naoh solution required for each and the average molarity of the naoh solution from the standardization trials with khp. you should report 3 significant figures, e. g. 0.488 m.
entry # vol acetic acid(ml) vol na0h(ml) m acetic acid
#1: 5.000 24.80
#2: 5.020 19.20
#3: 5.019 18.00

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, monsurviky
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Do you know the correct answer?
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized n...
Questions in other subjects:

Mathematics, 18.05.2021 20:40

English, 18.05.2021 20:40

Mathematics, 18.05.2021 20:40

English, 18.05.2021 20:40

Mathematics, 18.05.2021 20:40

Mathematics, 18.05.2021 20:40


Chemistry, 18.05.2021 20:40