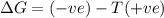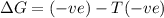Chemistry, 15.11.2019 02:31, williejaroid123
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spontaneity
a. δh is positive and δs is negative
b. δh is positive and δs is positive
c. δh is negative and δs is positive
d. δh negative and δs is negative
for a, b, c and d find out which of following they are:
1. spontaneous as written at all temperatures
2. spontaneous in reverse at all temperatures
3. spontaneous as written above a certain temperature
4. spontaneous as written below a certain temperature
they can only have one answer but 2 can have the same answer

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 06:00, womankrush538
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
Do you know the correct answer?
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spont...
Questions in other subjects:

Mathematics, 01.07.2019 03:40

History, 01.07.2019 03:40


Mathematics, 01.07.2019 03:40


Computers and Technology, 01.07.2019 03:40

Physics, 01.07.2019 03:40


Geography, 01.07.2019 03:40

Chemistry, 01.07.2019 03:40


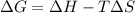
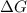 = Gibbs free energy
= Gibbs free energy 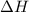 = enthalpy change
= enthalpy change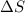 = entropy change
= entropy change 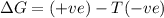
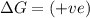
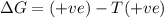
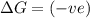 (at high temperature) (spontaneous)
(at high temperature) (spontaneous)