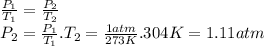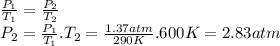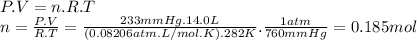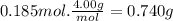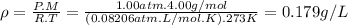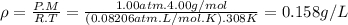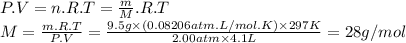Chemistry, 14.11.2019 23:31, victoriapellam04
One mole of an ideal gas is sealed in a 22.4-l container at a pressure of 1 atm and a temperature of 273 k. the temperature is then increased to 304 k , but the container does not expand. what will the new pressure be?
part a
the most appropriate formula for solving this problem includes only which variables?
enter the required variables, separated by commas (e. g., p, v,t).
q2)a sample of nitrogen gas in a 1.69-l container exerts a pressure of 1.37 atm at 17 ∘c.
-what is the pressure if the volume of the container is maintained constant and the temperature is raised to 327 ∘c?
q3)a gas mixture with a total pressure of 770 mmhgcontains each of the following gases at the indicated partial pressures: 120 mmhg co2, 227mmhg ar, and 190 mmhg o2. the mixture also contains helium gas
-what mass of helium gas is present in a 14.0-l sample of this mixture at 282 k ?
q4)
a
calculate the density of oxygen, o2, under each of the following conditions:
stp
1.00 atm and 35.0 ∘c
express your answers numerically in grams per liter. enter the density at stp first and separate your answers by a comma.
b
to identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 4.1-l bulb, then filled it with the gas at 2.00 atm and 24.0 ∘c and weighed it again. the difference in mass was 9.5 g . identify the gas.
express your answer as a chemical formula.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Do you know the correct answer?
One mole of an ideal gas is sealed in a 22.4-l container at a pressure of 1 atm and a temperature of...
Questions in other subjects:

History, 04.08.2019 18:40


Arts, 04.08.2019 18:40

Computers and Technology, 04.08.2019 18:40


Biology, 04.08.2019 18:40

Business, 04.08.2019 18:40

Mathematics, 04.08.2019 18:40