
Chemistry, 14.11.2019 22:31, avastanleyy
A255 ml round--bonom flask is weighed and found to have a mass of 114.85 g. a few millimeters of an easily vaporized liquid are added to the flask and the flask is immersed in a boiling water bath. all of the liquid vaporizes at the boiling tempentture of water, filling the flask with vapor. when all of the liquid has vaporized, the flask is removed from the bath, cooled, dried, and reweighed. the new mass of the flask and the condensed vapor is 115.23 g. which of the following compounds could the liquid be?
a. c₄h₁₀b. c₃h₇ohc. c₂h₆d. c₂h₅ohe. c₄h₉oh

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, dontcareanyonemo
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Do you know the correct answer?
A255 ml round--bonom flask is weighed and found to have a mass of 114.85 g. a few millimeters of an...
Questions in other subjects:



Mathematics, 21.02.2020 20:24




Chemistry, 21.02.2020 20:25




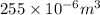 (as 1 ml =
(as 1 ml = 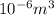 )
)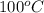 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K
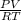
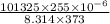
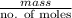
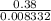
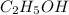 (molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).
(molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).




