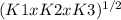Chemistry, 14.11.2019 22:31, HaJEReMY5170
Determine the value of the equilibrium constant, kgoal, for the reaction n2(g)+h2o(g)⇌no(g)+12n2h4(g), kgoal=? by making use of the following information: 1. n2(g)+o2(g)⇌2no(g), k1 = 4.10×10−31 2. n2(g)+2h2(g)⇌n2h4(g), k2 = 7.40×10−26 3. 2h2o(g)⇌2h2(g)+o2(g), k3 = 1.06×10−10express your answer numerically.
kgoal

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 10:00, isaiahromero15
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 10:30, villarrealc1987
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
Do you know the correct answer?
Determine the value of the equilibrium constant, kgoal, for the reaction n2(g)+h2o(g)⇌no(g)+12n2h4(g...
Questions in other subjects:

Mathematics, 28.06.2020 22:01

Mathematics, 28.06.2020 22:01




Mathematics, 28.06.2020 22:01



Mathematics, 28.06.2020 22:01