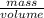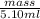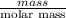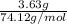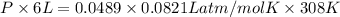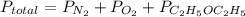Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, kellywelly82
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
Do you know the correct answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions in other subjects:


Mathematics, 08.02.2021 22:30




Mathematics, 08.02.2021 22:30

Mathematics, 08.02.2021 22:30


Mathematics, 08.02.2021 22:30

Arts, 08.02.2021 22:30


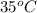 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K