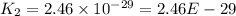Chemistry, 14.11.2019 06:31, strawberrymochi390
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be the rate constant at 119 k if the activation energy is 80. kj/mol? this is a second order reaction, giving k the units of m-1s-1 this will not change with the change in temperature. do not include units in your answer. exponential numbers need to be entered like this: 2 e-1 means 2 x 10-1. the rate constant, k, at 119 k equals:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3


Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1
Do you know the correct answer?
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be t...
Questions in other subjects:

Computers and Technology, 20.04.2021 19:10

Advanced Placement (AP), 20.04.2021 19:10

Mathematics, 20.04.2021 19:10





Physics, 20.04.2021 19:10


Mathematics, 20.04.2021 19:10


![Rate=1.4\times 10^{-2}[NO_2]^2](/tpl/images/0373/7605/5818c.png) ..........(1)
..........(1)![Rate=k[NO_2]^2](/tpl/images/0373/7605/d48ef.png) ............(2)
............(2)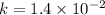
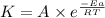
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0373/7605/6d953.png)
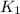 = rate constant at
= rate constant at 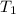 =
= 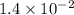
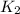 = rate constant at
= rate constant at 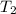 = ?
= ?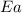 = activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole
= activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole![\log (\frac{K_2}{1.4\times 10^{-2}})=\frac{80000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{500}-\frac{1}{119}]](/tpl/images/0373/7605/44fe7.png)