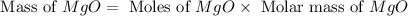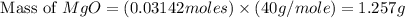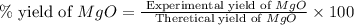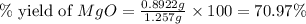Chemistry, 14.11.2019 06:31, yukichaniscool8
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only product, what would be the theoretical yield of the product?
2. if 0.8922 g of magnesium oxide is obtained from the reaction indicated in #1 above, what would be the percent yield of the magnesium oxide?
3. suppose the percent yield was calculated to be over 100%, what possible reasons could you give to account for it?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
Do you know the correct answer?
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only produ...
Questions in other subjects:

Social Studies, 04.02.2021 01:00


Mathematics, 04.02.2021 01:00

Physics, 04.02.2021 01:00



Mathematics, 04.02.2021 01:00

Biology, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00


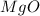 is, 1.257 grams.
is, 1.257 grams.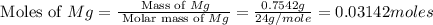
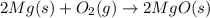
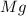 react to give 2 mole of
react to give 2 mole of