
Chemistry, 14.11.2019 01:31, ftshaechan
The zeisel method is an analytical procedure for determining the number of methoxyl groups in a compound. a weighed amount of the compound is heated with with concentrated hi, ether cleavage occurs, and the iodomethane product is distilled off and passed into an alcohol solution of agno3, where it reacts to form a precipitate of silver iodide. the agi is then collected and weighed, and the percentage of methoxyl groups in the sample is thereby determined.
1.06 g of vanillin yields 1.64 g of agi. if vanillin has a molecular weight of 152, how many methoxyl groups does vanillin contain?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Do you know the correct answer?
The zeisel method is an analytical procedure for determining the number of methoxyl groups in a comp...
Questions in other subjects:

History, 18.01.2020 05:31



History, 18.01.2020 05:31



Mathematics, 18.01.2020 05:31


Computers and Technology, 18.01.2020 05:31


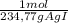 = 6,99x10⁻³ moles of AgI ≡ moles of methoxy groups.
= 6,99x10⁻³ moles of AgI ≡ moles of methoxy groups.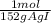 = 6,97x10⁻³ moles of vanilin
= 6,97x10⁻³ moles of vanilin




