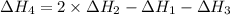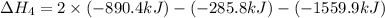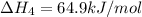1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9 kj/mol respectively at 298k.
equation 1 h2 + 1⁄2o2 > h2o δh = −285.8 kj
equation 2 ch4 + 2o2 > co2 + 2h2o δh = −890.4 kj
equation 3 c2h6 + 7⁄2o2 > 2co2 + 3h2o δh = −1559.9 kj
use the above equations to calculate (at the same temperature) the heat of reaction for the following reaction:
2ch4(g) > c2h6(g) + h2(g)
solution:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2


Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9...
Questions in other subjects:


Health, 16.10.2020 05:01

Computers and Technology, 16.10.2020 05:01


English, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

History, 16.10.2020 05:01


 ..[1]
..[1]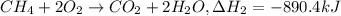 ..[2]
..[2]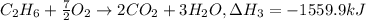 ..[3]
..[3]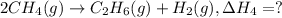 ..[4]
..[4]