Chemistry, 13.11.2019 06:31, PrincessKehlani15
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750 g sample that resulted from the oxidation of glucose and obtain 3.702 g co2 and 1.514 g h2o. how many carbon atoms are in the empirical formula of the isolated sample? (assume no other products present from the oxidation of glucose.)]

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
Do you know the correct answer?
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750...
Questions in other subjects:


Chemistry, 11.11.2020 04:30


English, 11.11.2020 04:30

Spanish, 11.11.2020 04:30

Mathematics, 11.11.2020 04:30


English, 11.11.2020 04:30



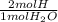 = 0.1682 mol H
= 0.1682 mol H