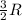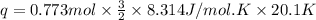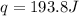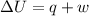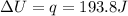A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough heat is applied to raise the temperature of the gas by 20.1 k. assuming ideal gas behavior, calculate the amount of heat in joules (q) required to affect this temperature change and the total change in internal energy, ? u. note that some books use ? e as the symbol for internal energy instead of ? u.
q= ? u=

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2


Chemistry, 23.06.2019 10:20, kyliemorgan8623
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
Do you know the correct answer?
A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough h...
Questions in other subjects:

English, 21.10.2020 01:01





History, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01

Computers and Technology, 21.10.2020 01:01


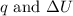 is 193.8 J and 193.8 J respectively.
is 193.8 J and 193.8 J respectively.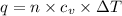
 = Change in temperature = 20.1 K
= Change in temperature = 20.1 K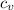 = heat capacity at constant volume of Xe (mono-atomic molecule) =
= heat capacity at constant volume of Xe (mono-atomic molecule) =