
Chemistry, 13.11.2019 05:31, SushiMagic
Propane is burned completely with excess oxygen. the product gas contains 24.8 mole% co2, 6.12% co, 40.8% h2o, and 28.6% o2.
a) calculate the percentage excess o2 fed to the furnace.
b) a student wrote the stiochemtric equation of the combustion of propane to form co2 and co as
2c3h8 + 17/2o2 > 3co2 + 3co +8h20
according to this equation, co2 and co should be a ratio of 1/1 in the reaction products, but in the product gas of part (a) they are in a ratio of 24.8/6.12. is the result possible? (hint: yes) explain how.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
Propane is burned completely with excess oxygen. the product gas contains 24.8 mole% co2, 6.12% co,...
Questions in other subjects:


Social Studies, 06.11.2020 07:10

Social Studies, 06.11.2020 07:10

Mathematics, 06.11.2020 07:10

Mathematics, 06.11.2020 07:10


Biology, 06.11.2020 07:10


Medicine, 06.11.2020 07:10

Mathematics, 06.11.2020 07:10


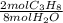 = 10,2 molesC₃H₈
= 10,2 molesC₃H₈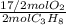 = 43,35 mol O₂
= 43,35 mol O₂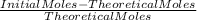 ×100
×100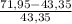 ×100 = 66% excess of O₂
×100 = 66% excess of O₂



