
Chemistry, 13.11.2019 04:31, friendsalwaysbae
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g) hexane is a low-boiling point component of gasoline. answer the question in part 1 through part 3, if the rate of reaction of c6h14 is 1.95 mol l-1s-1. the rate of reaction of c6h14 is the rate of disappearance of c6h14. what was the rate of formation of co2?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2



Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
Do you know the correct answer?
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g)...
Questions in other subjects:










Mathematics, 10.11.2020 22:10


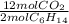 = 11,7 M/s
= 11,7 M/s



