
Choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. 0.075 m kno2 0.075 m licn 0.075 m (nh4)3po4 0.075 m nai 0.075 m nabro4

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Do you know the correct answer?
Choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvola...
Questions in other subjects:

Mathematics, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57



Mathematics, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57



English, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57


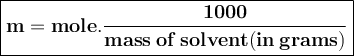
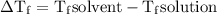

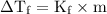
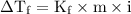
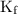 ) so that what affects the value of
) so that what affects the value of 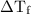 is the value of i
is the value of i




