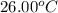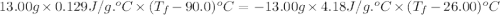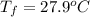Chemistry, 13.11.2019 00:31, amandamac7339
What is the final temperature of a system if 13.00 g of gold at 90.0°c is placed in 13.00 g of water at 26.00°c? the molar heat capacity of gold is 25.41 j/(mol · °c) and the heat capacity of water is 4.18 j/(g · °c).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
What is the final temperature of a system if 13.00 g of gold at 90.0°c is placed in 13.00 g of water...
Questions in other subjects:

Arts, 10.02.2021 19:30


SAT, 10.02.2021 19:30

Engineering, 10.02.2021 19:30

Chemistry, 10.02.2021 19:30




Mathematics, 10.02.2021 19:30

Computers and Technology, 10.02.2021 19:30


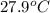
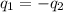
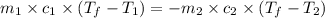
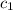 = specific heat of gold =
= specific heat of gold = 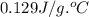
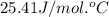
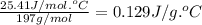
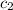 = heat capacity of water =
= heat capacity of water = 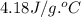
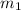 = mass of gold = 13.00 g
= mass of gold = 13.00 g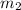 = mass of water = 13.00 g
= mass of water = 13.00 g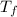 = final temperature of system = ?
= final temperature of system = ?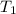 = initial temperature of gold =
= initial temperature of gold = 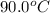
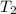 = initial temperature of water =
= initial temperature of water =