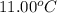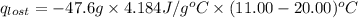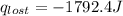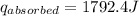A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water was 20.00°c. after the compound dissolved, the temperature of the water was 11.00°c. assume the heat was completely absorbed from the water and no heat was absorbed by the reaction container or the surroundings. calculate the heat absorbed by the process. the specific heat of water is 4.184 j/g·°c.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Do you know the correct answer?
A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water w...
Questions in other subjects:



History, 19.12.2019 06:31

Mathematics, 19.12.2019 06:31


Spanish, 19.12.2019 06:31

Spanish, 19.12.2019 06:31




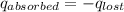
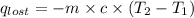
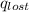 = heat lost by the water = ?
= heat lost by the water = ?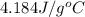
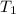 = initial temperature of water =
= initial temperature of water = 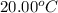
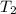 = final temperature of water =
= final temperature of water =