
Chemistry, 12.11.2019 23:31, maskoffvon
How many 3-liter balloons could the 12-l helium tank pressurized to 160 atm fill? keep in mind that an "exhausted" helium tank is not empty. in other words, once the gas inside the tank reaches atmospheric pressure, it will no longer be able to fill balloons. express the number of balloons to three significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
Do you know the correct answer?
How many 3-liter balloons could the 12-l helium tank pressurized to 160 atm fill? keep in mind that...
Questions in other subjects:



Mathematics, 22.12.2019 07:31





Mathematics, 22.12.2019 07:31

Mathematics, 22.12.2019 07:31


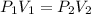
 = 636 balloons
= 636 balloons



