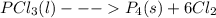Chemistry, 12.11.2019 06:31, gabrielolivas59
4pcl₃(l)  p₄(s) + 6cl₂(g); δh= 304.0 kj
p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown, which statement is true?
a) when 1 mol pcl₃(l) reacts, 304.0 kj are released. b) when 1 mol p₄(s) is produced, 304.0 kj are consumed. c) when 548.56 g pcl₃(l) react, 304.0 kj are released. d) when 137.14 g pcl₃(l) react, 304.0 kj are consumed. e) when 123.88 g p₄(s) are produced, 304.0 kj are released.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:00, kleathers97
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
Do you know the correct answer?
4pcl₃(l) [tex]\longrightarrow[/tex] p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown...
based on the reaction shown...
Questions in other subjects:



Mathematics, 29.09.2020 22:01

English, 29.09.2020 22:01


Mathematics, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01

Mathematics, 29.09.2020 22:01