
Chemistry, 12.11.2019 06:31, freedygotmoney
When an ion‑selective electrode for x+ was immersed in 0.0482 m xcl, the measured potential was 0.0460 v . what is the concentration of x+ when the potential is 0.0610 v ? assume that the electrode follows the nernst equation, the temperature is at 25 °c, and that the activity coefficient of x+ is 1.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Homepage10
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1


Do you know the correct answer?
When an ion‑selective electrode for x+ was immersed in 0.0482 m xcl, the measured potential was 0.04...
Questions in other subjects:

Mathematics, 10.07.2019 00:30


Social Studies, 10.07.2019 00:30



Mathematics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30

History, 10.07.2019 00:30


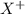 is 0.0861 M
is 0.0861 M![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{1}{[X]}](/tpl/images/0370/3205/84cb9.png)
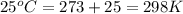
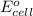 = standard electrode potential of the cell = ?
= standard electrode potential of the cell = ?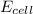 = emf of the cell = 0.0460 V
= emf of the cell = 0.0460 V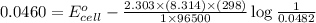
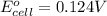
![0.0610=0.124-\frac{2.303\times (8.314)\times (298)}{1\times 96500}\log \frac{1}{[X]}](/tpl/images/0370/3205/50159.png)
![[X]=0.0861M](/tpl/images/0370/3205/21b33.png)





