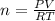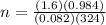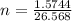Chemistry, 12.11.2019 04:31, ChloeLiz7111
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required to form 1.6 l of o2 at a temperature of 324 k and a pressure of 0.984 atm ?
express your answer using two significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
Do you know the correct answer?
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
Questions in other subjects:

Business, 18.08.2020 23:01


Mathematics, 18.08.2020 23:01