
Consider the acid/base properties of iron(iii). a. find the ph of a solution made by dissolving 0.7438 g iron(iii) chloride hexahydrate in 200.00 ml of water using [fe(h2o)6] 3+ (aq) ⇌ [fe(h2o)5oh] 2+ (aq) + h+ (aq) pka = 2.83 b. the iron(iii) can be made to precipitate from the solution by addition of hydroxide to form iron(iii) hydroxide. at what ph will the iron(iii) concentration be reduced to 1.0×10−10 m?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, alexisfaithsmith
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1
Do you know the correct answer?
Consider the acid/base properties of iron(iii). a. find the ph of a solution made by dissolving 0.74...
Questions in other subjects:

Mathematics, 24.04.2020 17:27



Mathematics, 24.04.2020 17:27





Advanced Placement (AP), 24.04.2020 17:27

Social Studies, 24.04.2020 17:27


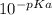
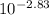
![Ka = \frac{[H^+]*[[Fe(H2O)5OH]^{+2}]}{[[Fe(H2O)6]^{+3}]}](/tpl/images/0369/9679/898a6.png)




